Written for practitioners in both the drug and biotechnology industries the Handbook of Analytical Validation carefully compiles current regulatory requirements on the validation of new or modified analytical methods. Sunday Feb 7 Details Save Extra with 4 offers. analytical method development and validation book.
Analytical Method Development And Validation Book, Analytical Method Development and Validation Michael E. 274 Santhosh Get al. What constitutes a validated method however is subject to analyst interpretation because there is no universally accepted industry practice for assay validation.
 Pdf Step By Step Analytical Methods Validation And Protocol In The Quality System Compliance Industry Semantic Scholar From semanticscholar.org
Pdf Step By Step Analytical Methods Validation And Protocol In The Quality System Compliance Industry Semantic Scholar From semanticscholar.org
Ad Über 7 Millionen englische Bücher. Development and Validation of Analytical Methods by Christopher Riley which are focused on explaining the concept from the scientific prospective. 67-83 Chantal Incledon.
In addition to the critical issues surrounding method validation this book also deals with other related factors such as method development data acquisition automation cleaning validation and regulatory considerations.
274 Santhosh Get al. Written for practitioners in both the drug and biotechnology industries the Handbook of Analytical Validation carefully compiles current regulatory requirements on the validation of new or modified analytical methods. Introduction Method validation is the process used to conf irm that the analytical procedure employed for a specific test is suitable for its intended use. Requirements to the analyst so as to enab le him to. Santhosh GNagasowjanya AAjitha YUma Maheswara Rao Department of Pharmaceutical Analysis and Quality Assurance CMR College of Pharmacy Medchal Road Kandlaykoya. Krull Editor 3 ratings See all formats and editions Kindle Edition 262578 Read with Our Free App Hardcover Paperback 48500 1 Used from 132500 10 New from 48500 Delivery by.
Another Article :

Next the process of validation is discussed starting with instrument qualification and concluding with system suitability. A REVIEW ON ANALYTICAL METHOD DEVELOPMENT AND VALIDATION Bhagyasree T1 Neelam I1 Ajitha A1 Uma Maheshwara Rao V1 Department of Pharmaceutical Analysis Quality Assurance CMR College of Pharmacy JNTU H University Hyderabad. Swartz Repost - Removed. Analytical Method Validation and Instrument Performance Verification. Introduction Method validation is the process used to conf irm that the analytical procedure employed for a specific test is suitable for its intended use. Pdf Development And Validation Of Rp Hplc Method For The Estimation Of Lisinopril In Tablet Dosage Form.

Analytical Method Development and Validation Michael E. To deepen ones knowledge the reader should choose the books eg. What constitutes a validated method however is subject to analyst interpretation because there is no universally accepted industry practice for assay validation. 2012-05-23 Analytical Method Development and Validation by Michael E. In Analytical Method Development and Validation the subject of developing and optimizing an HPLC method is presented culminating in a step-by-step guideline. Risk Based Test Method Development Validation And Life Cycle American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology.

Development and Validation of Plant-derived Medicines for Human. Santhosh GNagasowjanya AAjitha YUma Maheswara Rao Department of Pharmaceutical Analysis and Quality Assurance CMR College of Pharmacy Medchal Road Kandlaykoya. The need to validate an analytical or bioanalytical method is encountered by analysts in the pharmaceutical industry on an almost daily basis because adequately validated methods are a necessity for approvable regulatory filings. Development and Validation of Plant-derived Medicines for Human. Swartz Editor Ira S. Pdf Step By Step Analytical Methods Validation And Protocol In The Quality System Compliance Industry Semantic Scholar.
2009-03-15 Analytical Method Development and Validation by Michael E. What constitutes a validated method however is subject to analyst interpretation because there is no universally accepted industry practice for assay validation. Swartz Repost - Removed. Requirements to the analyst so as to enab le him to. HPLC Analytical method validation Pharmaceutical analysis Specificity Precision Accuracy. Analytical Method Transfer Usp 1224 Guideline Pharma Beginners.

Development and Validation of Plant-derived Medicines for Human. Krull Editor 3 ratings See all formats and editions Kindle Edition 262578 Read with Our Free App Hardcover Paperback 48500 1 Used from 132500 10 New from 48500 Delivery by. Krull Editor - Removed. Ad Über 7 Millionen englische Bücher. 2009-03-15 Analytical Method Development and Validation by Michael E. .

Analytical Method Validation and Instrument Performance Verification. To deepen ones knowledge the reader should choose the books eg. What constitutes a validated method however is subject to analyst interpretation because there is no universally accepted industry practice for assay validation. Written for practitioners in both the drug and biotechnology industries the Handbook of Analytical Validation carefully compiles current regulatory requirements on the validation of new or modified analytical methods. Ad Über 7 Millionen englische Bücher. Pdf Method Development And Validation Skills And Tricks.

HPLC Analytical method validation Pharmaceutical analysis Specificity Precision Accuracy. In Analytical Method Development and Validation the subject of developing and optimizing an HPLC method is presented culminating in a step-by-step guideline. This book is intended to serve as a guide to the analyst in terms of the issues and parameters that must be considered in the development and validation of analytical methods. Develop newer analytical methods for such drug s. Method Development and Validation of Analytical Procedures Kapil Kalra Dev Bhoomi Institute of Pharmacy an d Research Dehradun Uttarakhand India 1. Pin On Jocpr.

Given the need for generating reliable analytical data this book provides practical guidance for validating common and not-so-common analytical methods and for verifying the. Book Description Describes analytical methods development optimization and validation and provides examples of successful methods development and validation in high-performance liquid chromatography HPLC areas. A REVIEW ON ANALYTICAL METHOD DEVELOPMENT AND VALIDATION Bhagyasree T1 Neelam I1 Ajitha A1 Uma Maheshwara Rao V1 Department of Pharmaceutical Analysis Quality Assurance CMR College of Pharmacy JNTU H University Hyderabad. INTRODUCTION-PARTITION CHROMATOGRAPHY Partition chromatography can be subdivided into i l iquid-liquid chromatography and i i bonde d-phase chromatography. 2012-05-23 Analytical Method Development and Validation by Michael E. Method Development And Validation For Voglibose By Uplc Elsd Walmart Com In 2021 Development Method Book Format.
Ad Über 7 Millionen englische Bücher. Develop newer analytical methods for such drug s. 2012-05-23 Analytical Method Development and Validation by Michael E. To deepen ones knowledge the reader should choose the books eg. Swartz Repost - Removed. Hplc Method Development Ppt Download.

The required data for a. Method validation is defined as the process of pro ving that an analytical technique is acceptable for the intended use and this is an important. Development there is a need of method validation. 2012-05-23 Analytical Method Development and Validation by Michael E. 67-83 Chantal Incledon. Pin On Best Sample Template.
Sunday Feb 7 Details Save Extra with 4 offers. A REVIEW ON ANALYTICAL METHOD DEVELOPMENT AND VALIDATION Bhagyasree T1 Neelam I1 Ajitha A1 Uma Maheshwara Rao V1 Department of Pharmaceutical Analysis Quality Assurance CMR College of Pharmacy JNTU H University Hyderabad. Book Description Describes analytical methods development optimization and validation and provides examples of successful methods development and validation in high-performance liquid chromatography HPLC areas. Development there is a need of method validation. Analytical Procedures and Methods Validation for Drugs and Biologics Guidance for Industry. Analytical Method Validation Questions And Answers.
Krull Editor - Removed. A REVIEW ON ANALYTICAL METHOD DEVELOPMENT AND VALIDATION Bhagyasree T1 Neelam I1 Ajitha A1 Uma Maheshwara Rao V1 Department of Pharmaceutical Analysis Quality Assurance CMR College of Pharmacy JNTU H University Hyderabad. Krull Editor - Removed. Development and Validation of Plant-derived Medicines for Human. Development there is a need of method validation. Analytical Method Validation Questions And Answers.

Ebooks list page. Given the need for generating reliable analytical data this book provides practical guidance for validating common and not-so-common analytical methods and for verifying the. Books on Google Play Analytical Method Development and Validation Michael E. Ebooks list page. Requirements to the analyst so as to enab le him to. Development And Validation Of Liquid Chromatography Rp Hplc Methodology For Estimation Of Efonidipine Hcl Chemistry Help Teaching Chemistry Science Chemistry.
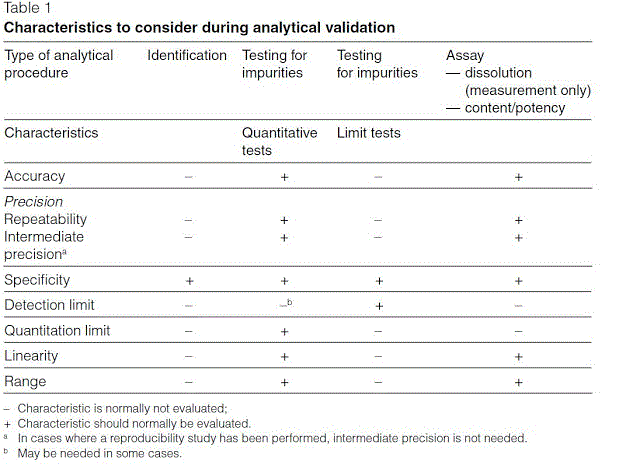
A REVIEW ON ANALYTICAL METHOD DEVELOPMENT AND VALIDATION Bhagyasree T1 Neelam I1 Ajitha A1 Uma Maheshwara Rao V1 Department of Pharmaceutical Analysis Quality Assurance CMR College of Pharmacy JNTU H University Hyderabad. This book is intended to serve as a guide to the analyst in terms of the issues and parameters that must be considered in the development and validation of analytical methods. Food and Drug Administration. Development there is a need of method validation. Development and Validation of Automated Methods Pages. Analytical Method Validation Pharmaceutical Guidelines.

Shedding light on method validation from a practical standpoint the handbook. In Analytical Method Development and Validation the subject of developing and optimizing an HPLC method is presented culminating in a step-by-step guideline. 2012-05-23 Analytical Method Development and Validation by Michael E. Method validation is defined as the process of pro ving that an analytical technique is acceptable for the intended use and this is an important. Krull Editor 3 ratings See all formats and editions Kindle Edition 262578 Read with Our Free App Hardcover Paperback 48500 1 Used from 132500 10 New from 48500 Delivery by. Performance Parameters For Analytical Method Validation Controversies And Discrepancies Among Numerous Guidelines Sciencedirect.









